Rintodestrant (G1T48), an oral selective estrogen receptor degrader, in combination with palbociclib for ER+/HER2– advanced breast cancer: phase 1 results
Marina Maglakelidze1; Iurie Bulat2; Dinara Ryspayeva3; Boris Krastev4; Maia Gogiladze1; Adrian Crijanovschi2; Philippe Aftimos5; Patrick Neven6; Mark Pegram7; Catharina Willemien Menke-van der Houven Van Oordt8; E. Claire Dees9; Carolien Schröder10; Agnes Jager11; Linnea Chap12; Erika Hamilton13; Massimo Cristofanilli14; Susanna Ulahannan15; Jorianne Boers10; Ramsha Iqbal8; and Sarika Jain16
This presentation is the intellectual property of the author/presenter. Copies of this poster obtained through QR (Quick Response) and/or text key codes are for personal use only and may not be reproduced without written permission of the authors. Contact them at marina.maglakelidze@arensia-em.com or sjain@g1therapeutics.com for permission to reprint and/or distribute.
1 LLC Arensia Exploratory Medicine, Tblisi, Georgia; 2 Arensia Exploratory Medicine Research Unit, Institute Of Oncology, Chisinau, Moldova; 3 Arensia Exploratory Medicine Research Unit, National Cancer Institute, Kyiv, Ukraine; 4 MHAT Hospital For Women Health Nadezhda, Sofia, Bulgaria; 5 Institut Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium; 6 UZ Leuven, Leuven, Belgium; 7 Stanford Women’s Cancer Center, Stanford, CA, USA; 8 Amsterdam UMC, Vrije Universiteit Medical Center, Amsterdam, the Netherlands; 9 UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA; 10 University Medical Center Groningen, Groningen, the Netherlands; 11 Erasmus MC Cancer Institute, Rotterdam, the Netherlands; 12 Beverly Hills Cancer Center, Beverly Hills, CA, USA; 13 Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN, USA; 14 RH Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA; 15 Stephenson Cancer Center, Oklahoma City, OK, USA; 16 G1 Therapeutics, Inc., Research Triangle Park, NC, USA
American Society of Clinical Oncology Annual Meeting | June 4–8, 2021 | Chicago, IL, USA
Background
- Rintodestrant is a potent oral selective estrogen receptor degrader (SERD) that competitively binds to the estrogen receptor (ER) and blocks ER signaling in tumors resistant to other endocrine therapies (ETs)1,2
- Study G1T48-01 (NCT03455270) comprises 3 parts: dose escalation of monotherapy rintodestrant (part 1), dose expansion of monotherapy rintodestrant (part 2), and rintodestrant in combination with palbociclib therapy (part 3)
- Results from parts 1 and 2 showed a favorable safety profile and antitumor activity with once-daily (QD) rintodestrant in patients with heavily pretreated ER-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC), including those with tumors harboring pathogenic ESR1 variants3–5
- The optimal dose of rintodestrant was selected to be 800 mg QD
- Here, we present part 3 combining rintodestrant with palbociclib, an oral cyclin-dependent kinase (CDK) 4/6 inhibitor
Methods
- This phase 1, first-in-human, open-label study is evaluating rintodestrant in women with ER+/HER2– ABC after progression on ET3,4
- Part 3: continuous rintodestrant 800 mg QD combined with palbociclib 125 mg QD for 21 days in 28-day cycles (data cut: April 7, 2021)
- Key inclusion criteria:
- Female patients ≥ 18 years of age
- Histological/cytological confirmation of ER+/HER2– ABC
- ≥ 24 months of ET in the adjuvant setting before recurrence or progression OR ≥ 6 months of ET in the advanced/metastatic setting before progression
- ≤ 1 prior line each of ET or cytotoxic chemotherapy in the metastatic setting
- Prior CDK4/6 inhibitor therapy, investigational oral SERDs, or selective ER covalent antagonists in any setting were prohibited
- Prior everolimus therapy was allowed
- Eastern Cooperative Oncology Group performance status: 0 or 1
- Primary objectives: safety and tolerability of rintodestrant with palbociclib
- Secondary objectives: antitumor activity per RECIST v1.1, including best overall response, clinical benefit rate in measurable and nonmeasurable disease (as defined by percentage of patients with either confirmed complete or partial response [PR], or stable disease lasting ≥ 24 weeks), progression-free survival (PFS), overall survival, and palbociclib and rintodestrant pharmacokinetics
- Exploratory objectives: mutation profiling (cell-free DNA [cfDNA]), change in ER expression from baseline to 6-week on-treatment tumor biopsies (cycle [C] 2 day [D] 15), and assessment of UGT1A1 genetic polymorphisms
Results
BASELINE CHARACTERISTICS
- Median (range) number of prior lines of therapy in the advanced setting was 1 (0–2), including chemotherapy (48%) and fulvestrant (15%; Table 1)
- Median (range) number of prior lines of ET in the advanced setting was 1 (0–1),
with 73% of patients having received 1 prior line; 65% of patients received ET in
the adjuvant setting and relapsed while on or within 12 months of completing
adjuvant therapy
- Forty-five percent of patients had received both ET and chemotherapy in the advanced setting
- Forty percent of patients had liver metastasis, and 30% had lung metastasis
- All patients had ER+ disease; 90% had tumors defined as high-ER (ER > 10%), 8% as low-ER (ER = 1–10%), and 13% had progesterone receptor-negative ABC
TABLE 1. BASELINE CHARACTERISTICS
SAFETY AND TOLERABILITY
- Safety data are summarized in Table 2; 98% of patients experienced ≥ 1 treatment-emergent adverse event (TEAE)
- Three patients (8%) experienced a rintodestrant-related TEAE; all were grade 2 and included neutropenia (3%), nausea (3%), and vomiting (3%)
- Hot flashes, diarrhea, and fatigue were the most common rintodestrant-related TEAEs reported in parts 1 and 2 (monotherapy)4; in patients treated with combination rintodestrant and palbociclib, 1 case (3%) each of diarrhea and fatigue were reported, but neither were considered related to rintodestrant/palbociclib
- One patient (3%) experienced the serious adverse event (SAE) of grade 2 COVID-19 pneumonia, considered by the investigator to be related to palbociclib
- No rintodestrant-related SAEs or dose reductions were reported
- Palbociclib dose reductions were reported in 9 patients (23%; 8 patients [20%] due to grade 3/4 neutropenia and 1 patient [3%] due to grade 3 febrile neutropenia); palbociclib dose delays were reported in 19 patients (48%)
- No discontinuations or deaths due to TEAEs were reported
TABLE 2. TEAEs REPORTED REGARDLESS OF CAUSALITY
CLINICAL ACTIVITY
- Best overall response and clinical benefit rate are summarized in Table 3 and Figure 1
- Median PFS was 7.4 months (95% CI: 3.7–not reached); data not yet mature
- Median (range) duration of treatment for rintodestrant and palbociclib was 6.2 (1.5–8.5) months (Figure 1)
- One patient with confirmed PR had ABC harboring ESR1 and PIK3CA variants at baseline and had received prior treatment with fulvestrant
TABLE 3. BEST OVERALL RESPONSE
PHARMACOKINETICS AND PHARMACODYNAMICS
- Rintodestrant exposure levels were as predicted; palbociclib steady-state trough concentrations were similar to historical data6 (geometric mean 61.7 ng/mL vs 60.8 ng/mL, respectively), indicating that rintodestrant had no impact on palbociclib pharmacokinetics
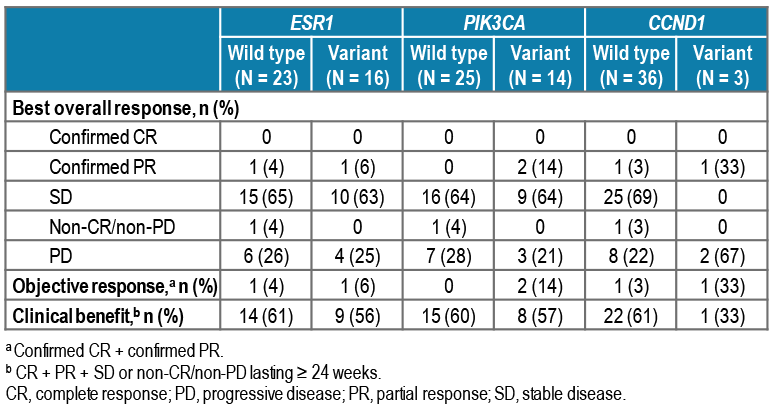
- Of 39 patients tested for baseline cfDNA, 41% had tumors harboring ≥ 1 ESR1 variant, 36% ≥ 1 PIK3CA variant, and 28% ≥ 1 ESR1 and ≥ 1 PIK3CA variant (Table 4 and Figure 2)
- Of 14 patients with detectable ESR1 or PIK3CA variants at baseline and evaluable samples at C1D15, all patients (100%) whose tumors harbored ESR1 variants had a decrease in mean variant allele frequency (mVAF), and 10 patients (71%) that harbored PIK3CA variants had a decrease in mVAF (Figure 3)
- A decrease in mVAF at C1D15 was observed in 29 of 33 patients (88%) with evaluable samples at baseline and C1D15 (Figure 3)
- Of the 2 patients who had confirmed PR, 1 patient had 2 ESR1 variants (D538G and Y537N) and 1 PIK3CA variant (N345K), and the other patient had a single PIK3CA variant (E545K) (Figure 1 and Table 5)
TABLE 4. ESR1, PIK3CA, AND CCND1 VARIANTS, AND DISEASE CHARACTERISTICS
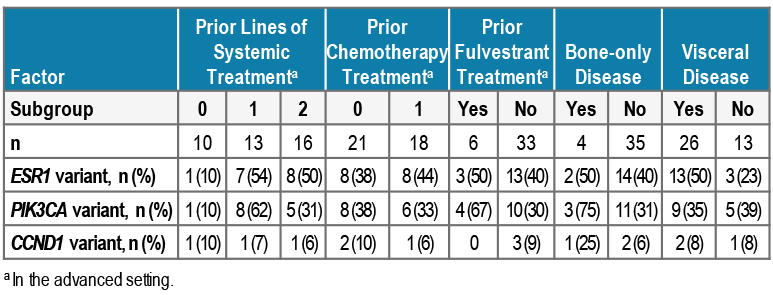
FIGURE 1. (A) TREATMENT DURATION AND RESPONSE, AND (B, C) CHANGE FROM BASELINE IN TUMOR SIZE FOR TARGET LESIONS
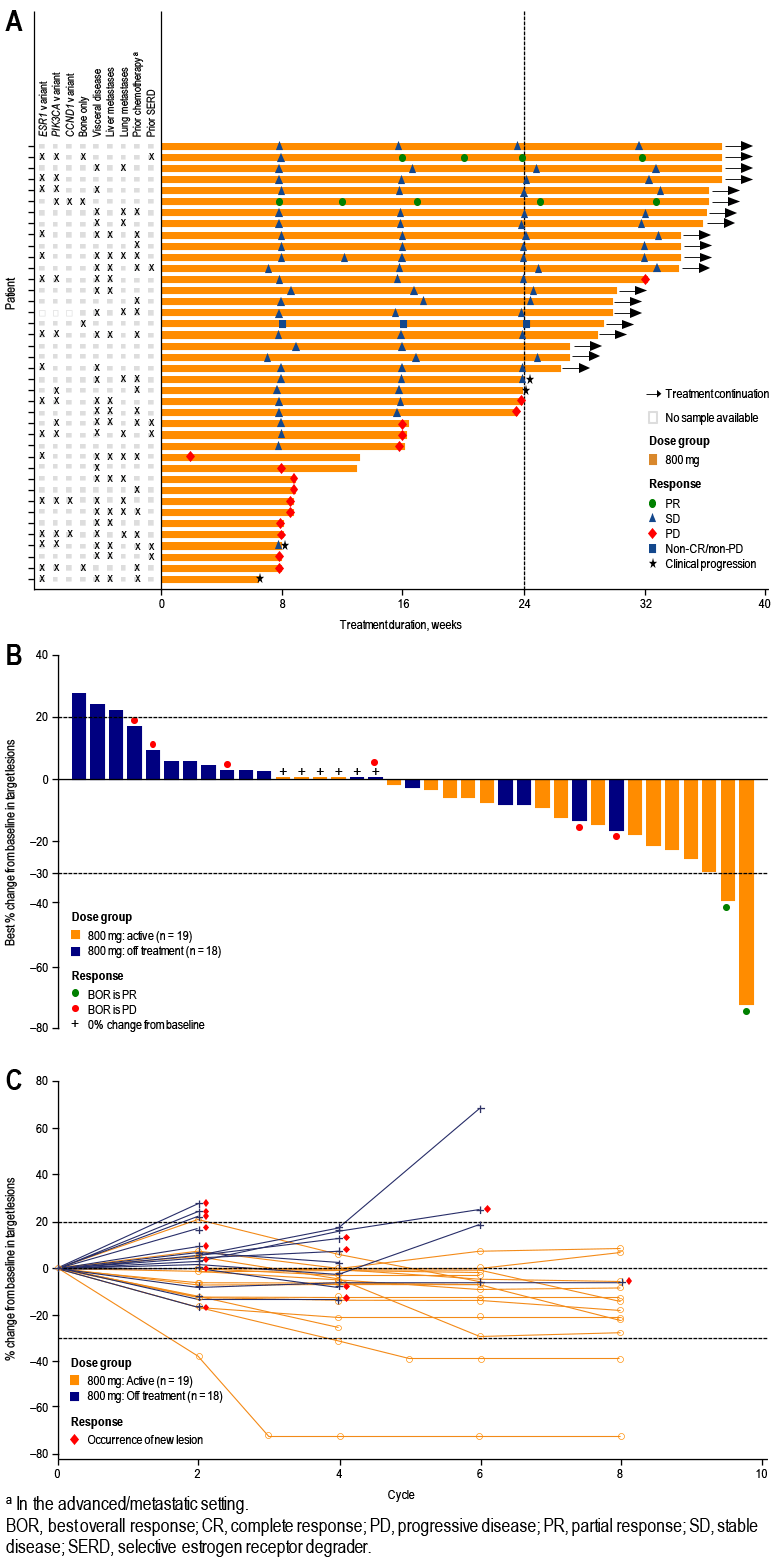
FIGURE 2. ESR1 AND PIK3CA VARIANTS
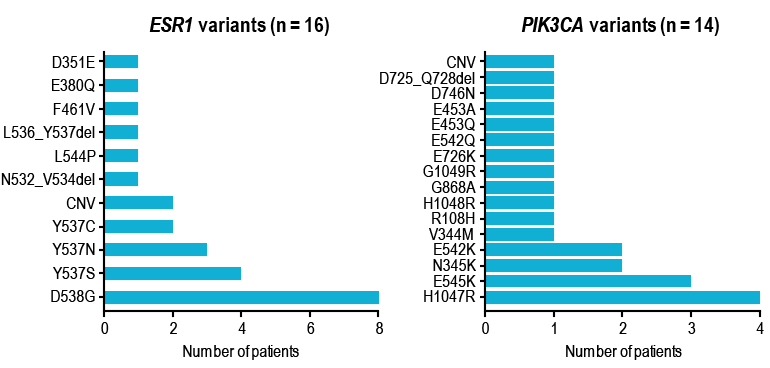
FIGURE 3. mVAF CHANGES AT C1D1 VS C1D15
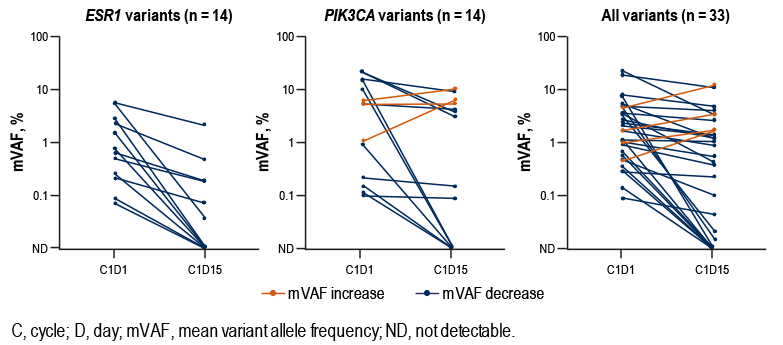
TABLE 5. ESR1, PIK3CA, AND CCND1 VARIANTS AT BASELINE, AND BEST OVERALL RESPONSE
Conclusions
- Rintodestrant combined with palbociclib was very well tolerated
- Most common TEAEs of neutropenia and leukopenia are consistent with the known safety profile of palbociclib, as reported in PALOMA-36
- Addition of rintodestrant to palbociclib did not result in additional or more severe toxicities, in particular, nausea, vomiting, or diarrhea
- Encouraging antitumor activity was observed (data not yet mature)
- The clinical benefit rate doubled from 30%4 to 60% when palbociclib was added to rintodestrant, suggesting favorable antitumor activity in patients with ER+/HER2– ABC, including in patients with tumors harboring ESR1 variants
References and Acknowledgments
REFERENCES
- 1. Nathan MR, Schmid P. Oncol Ther. 2017;5:17–29.
- 2. Robertson JFR, et al. Clin Breast Cancer. 2014;14:381–9.
- 3. Dees EC, et al. Ann Oncol. 2019;30(Suppl 5):v104–42.
- 4. Aftimos P, et al. SABCS 2020; poster PS12-04.
- 5. Aftimos P, et al. SABCS 2020; poster PD8-07.
- 6. IBRANCE® (palbociclib). United States Prescribing Information, July 2020.
ACKNOWLEDGMENTS
- We thank all the investigators and site staff, with special thanks to the patients and their families
- We thank G1 Therapeutics team members who supported the creation of this poster
- Study sponsored by G1 Therapeutics. Editorial assistance was provided by Alligent Europe (Envision Pharma Group), funded by G1 Therapeutics