Understanding hematological adverse event management through health care resource utilization, costs, and treatment patterns of patients with extensive-stage small cell lung cancer treated in the community oncology setting
Jerome Goldschmidt1; Alisha Monnette2; Ping Shi2; Divea Venkatasetty2; Huan Huang3; and Marc Chioda3
This presentation is the intellectual property of the author/presenter. Copies of this poster obtained through QR (Quick Response) and/or text key codes are for personal use only and may not be reproduced without written permission of the authors. Contact them at hhuan@g1therapeutics.com for permission to reprint and/or distribute.
1 US Oncology Network, Blacksburg, VA; 2 Ontada, Woodlands, TX; 3 G1 Therapeutics, Inc., Research Triangle Park, NC
Introduction
- Myelosuppressive hematological adverse events (HAEs; anemia, neutropenia, and/or thrombocytopenia) are common complications of chemotherapy among patients with cancer1
- Cytotoxic chemotherapy remains the cornerstone of treatment for extensive-stage small cell lung cancer (ES-SCLC)2–4
Objective
- To assess health care resource utilization (HCRU), costs, and treatment patterns associated with myelosuppressive HAEs among patients with ES-SCLC treated with chemotherapy in the community oncology setting
Methods
DATA SOURCE
- This retrospective observational study was conducted using structured data from The US Oncology Network's iKnowMed electronic health record system
- Data on vital status from the Social Security Administration's Limited Access Death Master File and HCRU data from the Financial Data Warehouse were included
STUDY POPULATION
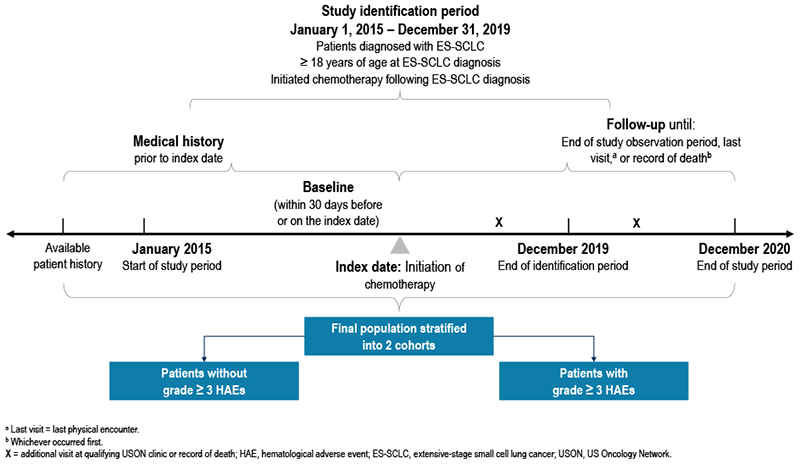
- Adult patients with ES-SCLC who initiated chemotherapy between January 1, 2015, and December 31, 2019, were stratified into 2 study cohorts on the basis of the presence of grade ≥ 3 HAEs after chemotherapy initiation (index date; Figure 1)
- The cohort with grade ≥ 3 HAEs comprised patients who had ≥ 1 of the following events after index: grade ≥ 3 anemia (hemoglobin <8.0 g/dL), grade ≥ 3 neutropenia (absolute neutrophil count <1000/μL), or grade ≥ 3 thrombocytopenia (platelets <50,000/μL)5
- The cohort without grade ≥ 3 HAEs comprised patients who had no grade ≥ 3 anemia, grade ≥ 3 neutropenia, or grade ≥ 3 thrombocytopenia events after index
- The first course of chemotherapy initiated after diagnosis of ES-SCLC was defined as chemotherapy initiation; patients must have had no evidence of receiving any chemotherapy within the 12 months prior to diagnosis
- Patients were followed longitudinally from the index date until December 31, 2020, death, or the last patient record, whichever occurred first
- Patients diagnosed with other primary tumors or enrolled in clinical trials during the study period were excluded
OUTCOME AND ANALYSIS
- HAEs were identified using laboratory values from iKnowMed on the basis of Common Terminology Criteria for Adverse Events version 5.0 definitions5
- The prevalence and frequency of HAEs (by type and grade), treatment patterns, HCRU (including supportive care utilization [granulocyte colony-stimulating factor {G-CSF}, erythropoiesis-stimulating agents {ESAs}, intravenous {IV} hydration]), and health care costs during the follow-up period were evaluated for both cohorts. Costs were adjusted to the year 20216
FIGURE 1. STUDY DESIGN OVERVIEW
Results
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
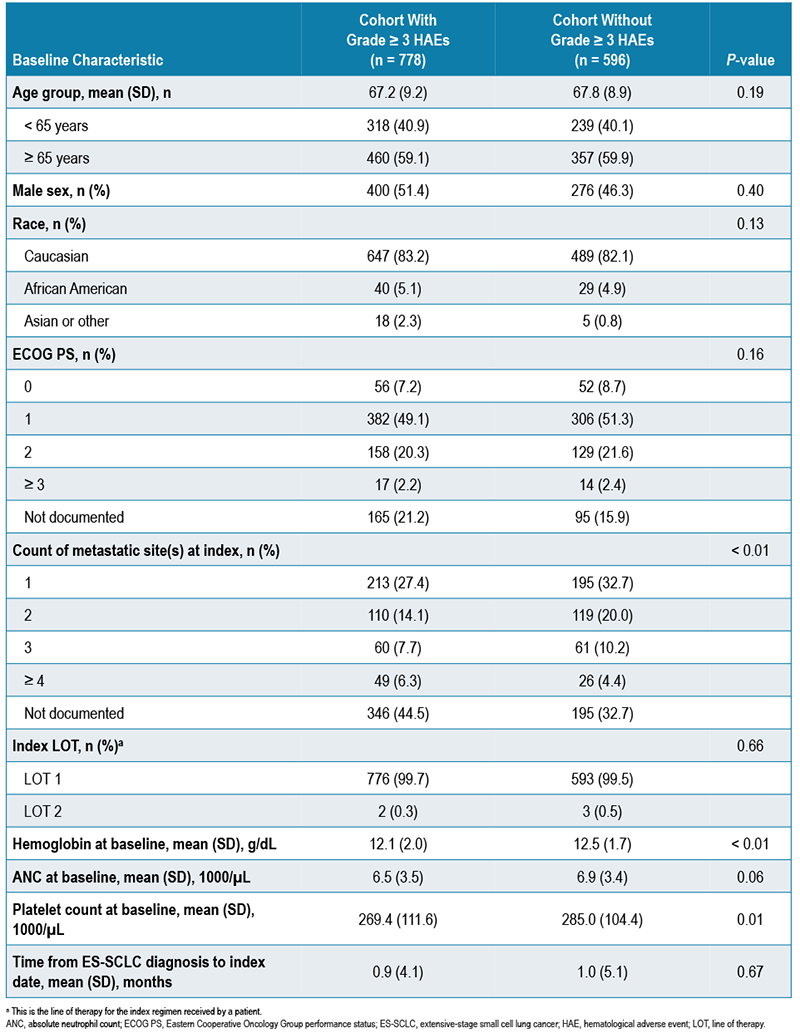
- The study population included 778 patients who had ≥ 1 grade ≥ 3 HAE and 596 patients who did not have a grade ≥ 3 HAE after chemotherapy initiation. Demographic and clinical characteristics at baseline are shown in Table 1
TABLE 1. DEMOGRAPHIC AND CLINICAL CHARACTERISTICS AT BASELINE
MYELOSUPPRESSIVE EVENTS
- Among 778 patients in the grade ≥ 3 HAE cohort, 47.3% of patients had grade ≥ 3 anemia, 58.9% had grade ≥ 3 neutropenia, and 37.3% had grade ≥ 3 thrombocytopenia within 12 months post index
- Mean numbers of events within 12 months post index were 2.0, 1.8, and 2.4 for patients who experienced grade ≥ 3 anemia, grade ≥ 3 neutropenia, and grade ≥ 3 thrombocytopenia, respectively
- 12.2% of patients with grade ≥ 3 HAEs had evidence of major bleeding events (platelets < 20,000/μL)
- Grade ≥ 3 leukopenia and lymphopenia events also occurred in a notable number of patients (Table 2)
TREATMENT PATTERNS
- Almost all patients (> 99%) received first-line chemotherapy at index (approximately 80% received a platinum-/etoposide-containing regimen and 15% received platinum/etoposide in combination with immunotherapy) in both cohorts
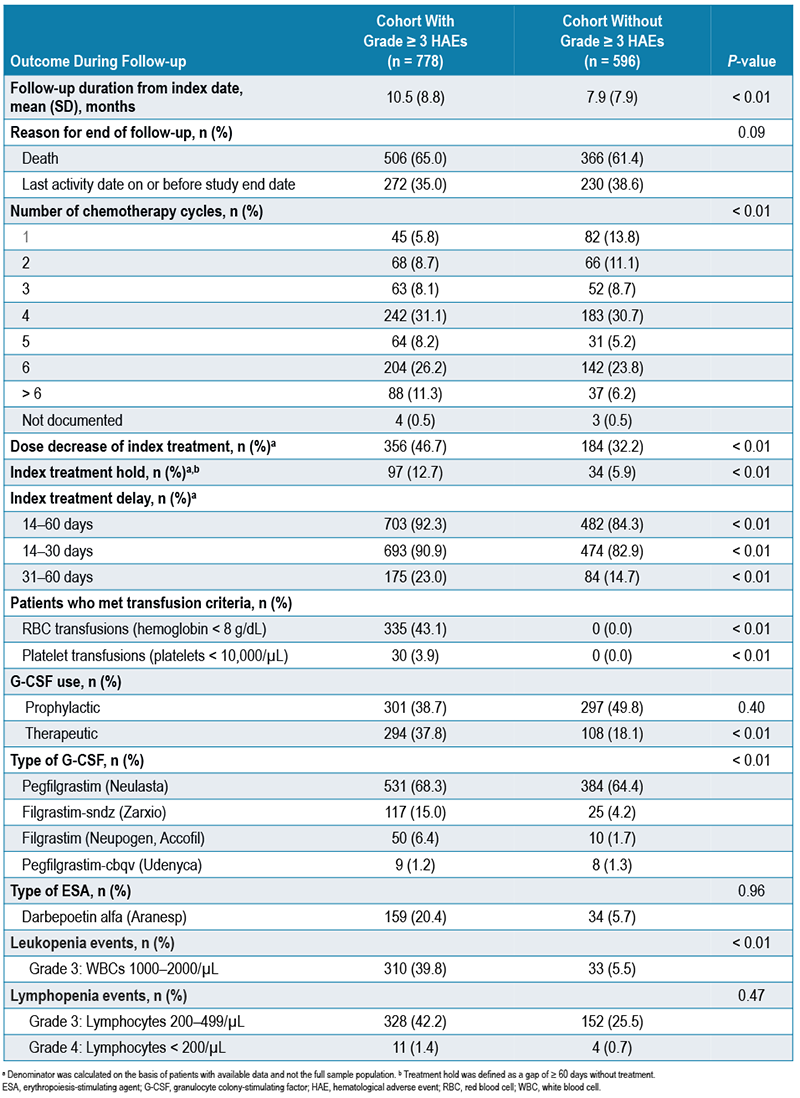
- Patients with grade ≥ 3 HAEs had a higher proportion of dose reductions (46.7% vs 32.2%), treatment holds (12.7% vs 5.9%), and treatment delays between 14–60 days (92.3% vs 84.3%) after chemotherapy initiation (all P < 0.001) compared with patients without grade ≥ 3 HAEs (Table 2)
TABLE 2. TREATMENT OUTCOMES DURING FOLLOW-UP
HCRU FOR HAE MANAGEMENT
- 43.1% of patients with grade ≥ 3 HAEs were eligible for RBC transfusions and 3.9% were eligible for platelet transfusions as indicated by laboratory values (Table 2)
- Patients with grade ≥ 3 HAEs were more likely to receive therapeutic G-CSF than patients without grade ≥ 3 HAEs (37.8% vs 18.1%; P <0.01), and prophylactic G-CSF use was similar among both cohorts (38.7% vs 49.8%; P = 0.40; Table 2, Figure 2)
- Receiving G-CSF within 1–3 days after chemotherapy initiation was considered prophylactic use
- Receiving G-CSF ≥ 4 days after chemotherapy initiation was considered therapeutic use
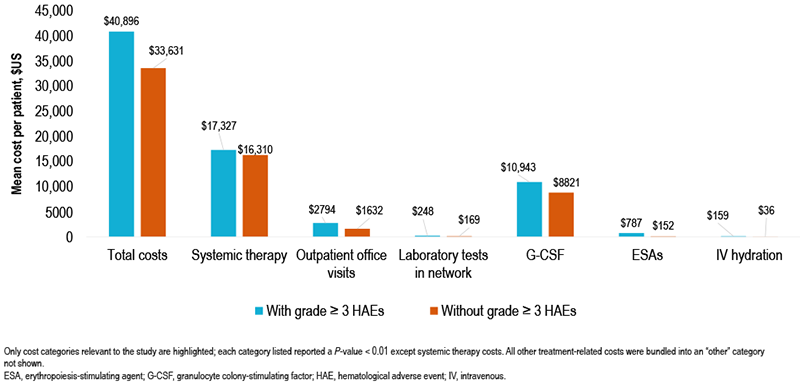
- The total costs within 12 months post index were higher for patients with grade ≥ 3 HAEs than for those without ($40,896 vs $33,631; P < 0.01; Figure 3)
- Patients with grade ≥ 3 HAEs had a mean of 10.7 outpatient visits within 12 months post index, versus 7.7 outpatient visits for those without grade ≥ 3 HAEs (P < 0.01; Table 3)
- Compared with patients without grade ≥ 3 HAEs, patients with grade ≥ 3 HAEs also had greater:
- G-CSF use (64.1% vs 57.2%; mean number of administrations 3.5 vs 2.4; mean cost per patient $10,943 vs $8821; all P < 0.01; Table 3, Figure 3) within 12 months after the index date
- ESA use (18.6% vs 4.9%; mean number of administrations 0.7 vs 0.1; mean cost per patient $787 vs $152; all P < 0.01; Table 3, Figure 3) within 12 months after the index date
- IV hydration use (46.0% vs 30.4%; mean number of administrations 2.3 vs 1.2; mean cost per patient $159 vs $36; all P < 0.01; Table 3, Figure 3) within 12 months after the index date
FIGURE 2. G-CSF USE IN PATIENTS WITH AND WITHOUT GRADE ≥ 3 HAEs
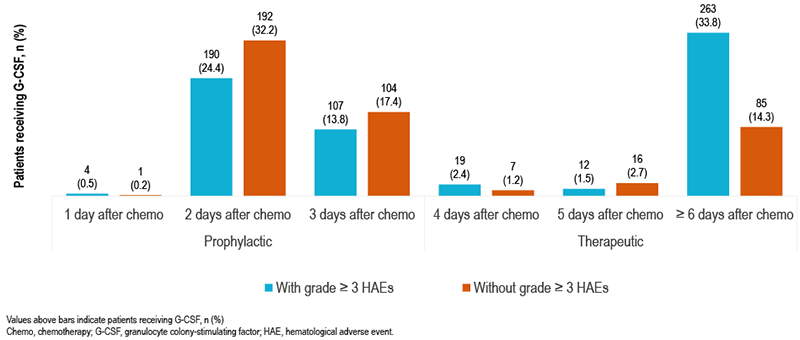
TABLE 3. HCRU WITHIN 12 MONTHS AFTER THE INDEX DATE – PER PATIENT
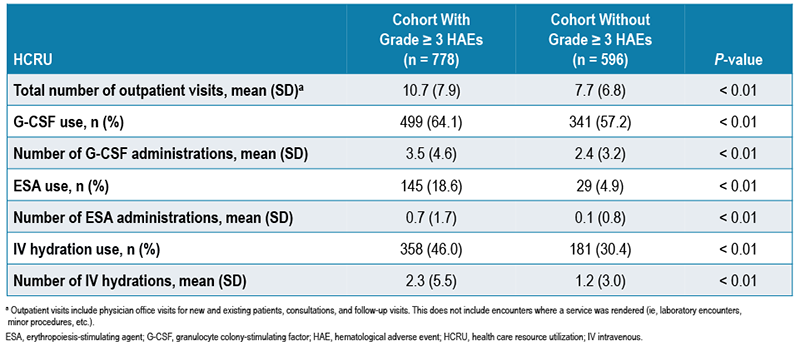
FIGURE 3. HEALTH CARE COSTS WITHIN 12 MONTHS AFTER THE INDEX DATE – PER PATIENT
Limitations
- Results were based on data from community oncology settings and may not be generalizable beyond this setting
- Data in the inpatient settings were not captured; inpatient costs or costs for transfusions were not included
Conclusions
- The results suggest there is significant burden of myelosuppressive HAEs on patients with ES-SCLC in a community oncology setting
- Patients with grade ≥ 3 HAEs had more dose reductions, treatment delays, and HCRU than those without grade ≥ 3 HAEs
- Therapies to protect bone marrow from multilineage HAEs have the potential to reduce such burden. Future research should investigate HCRU and cost burden in the inpatient setting to better understand the full scope of HAE management
References and Acknowledgments
REFERENCES
- 1. Kurtin S. J Adv Pract Oncol. 2012;3:209–24.
- 2. Aapro M, et al; ESMO Guidelines Committee. Ann Oncol. 2018;29(suppl 4):iv96–110.
- 3. Crawford J, et al. Support Care Cancer. 2020;28:925–32.
- 4. Kuter DJ. Oncology (Williston Park). 2015;29:282–94.
- 5. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. US Department of Health and Human Services: NIH. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed September 22, 2021.
- 6. Bureau of Labor Statistics. Medical Care Consumer Price Index. https://www.bls.gov/cpi/factsheets/. Accessed October 7, 2021.
ACKNOWLEDGMENTS
- Study sponsored by G1 Therapeutics.